“We bought an Arcam S12 electron beam melting machine back in 2006,” he recalled, noting that efforts at North Carolina State University (NCSU), which installed the first S12, enabled the machine’s ability to print titanium and opened the door for titanium-AM medical applications at Medical Modeling. “(NCSU was) doing cool work and I thought, ‘gosh, this is amazing!.’ We do a lot of personalized things, and here’s a machine that allows us to make a metal part on a one-off basis. This is perfect.”
 Considering that someone had to prove the technology out for
medical applications, Medical Modeling bought the S12 and set to work.
Considering that someone had to prove the technology out for
medical applications, Medical Modeling bought the S12 and set to work.
“Going from plastics to metals was not trivial, and it was so expensive, especially with all the other kit that’s needed,” Christensen said. “We brought on a metallurgist and the smart people on our staff helped figure things out.”
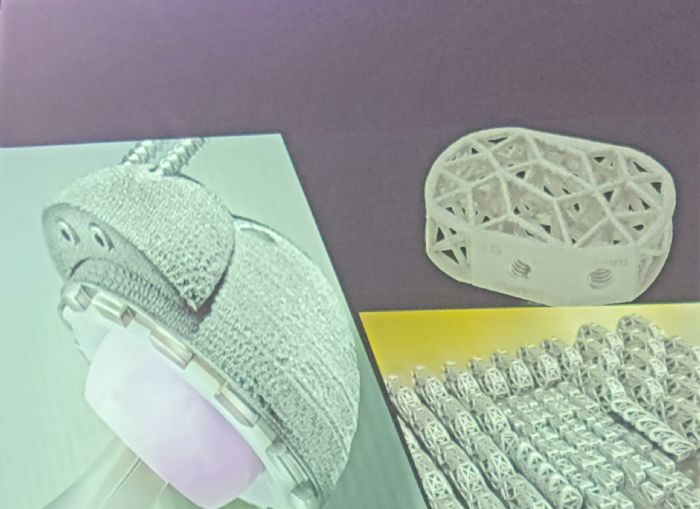
Medical Modeling did figure things out, producing the first FDA-cleared 3D-printed titanium part—a hip cup for orthopedic-surgery applications—in 2010 for Exactech in Gainesville, FL. Medical Modeling also produced the first FDA-cleared titanium spinal cages for Texas-based 4Web Medical in 2011.
With these successes, Christensen realized the possibility
of a solid business case for personalized production of such implantable
devices.
“I could see it, but just couldn’t make it work,” he told Grimm. “Today, there's a story there that keeps getting better. It has taken 10 or 15 yr., and most of the business still is making off-the-shelf devices in assorted sizes. Patient-specific devices still is an emerging application. It's there and is being proved, but some of the early promises haven’t been delivered.
“Many head, neck and facial surgeries,” he continued, “use personalized tools and plans, models, guides, and implants. But, orthopedics is a much bigger market. Orthopedic applications for personalization, while still a small segment, should get much bigger.”
Christensen also discussed efforts to better-standardize
reimbursement for planning, modeling and implanting work, and related
consultation with medical professionals.
“I could never figure out the reimbursement puzzles for what we were providing the surgeons in hospitals,” he explained. “We tried three times to get nomenclature established through the American Medical Association for reimbursement coding, and finally decided to forget it.”
Temporary codes recently have been established, Christensen
reported as a current working member on teams seeking such coding.
“I'm really excited to be playing a role in seeing this come to fruition,” he said. “It’s been needed for 20 yr.—amazing that it’s still not done.”
Concluding his Innovators Showcase interview with Grimm,
Christensen dispensed some advice for AM startups and entrepreneurs.
“For a small business, I have some keys,” he said. “First, cash is king. You need to be solvent, and you need to collect your payments properly. Accounts receivables are very important. Second, consider leverage. As a small company, you’re always struggling for leverage—trying to work with much bigger companies without having leverage. When you get leverage, hold on to it and use it.”
Also, don’t spread out by taking on too many projects.
“Small companies just say yes to everything just to try getting money coming in,” Christensen offered. “When looking for that killer application, you want to say yes to everything, but you have to pare it down. To a certain degree, you have to in some ways hold yourself back, and pick some bets to go deeper. Even when you think you should be looking at 500 things, pare down to the four things that actually have some chance to succeed.”
See also: 3D Systems, Materialise USA LLC
Technologies:
 At AMUG...During the Additive Manufacturing Users Group (AMUG) April 6 sessions, Todd Grimm, president of T.A. Grimm & Associates, Inc., hosted the seventh annual Innovators Showcase, an on-stage fireside chat where attendees get to know an innovator in the industry and discover insights from that individual’s experiences. This year’s subject, Andy Christensen, is founder and past president of Medical Modeling Inc.
At AMUG...During the Additive Manufacturing Users Group (AMUG) April 6 sessions, Todd Grimm, president of T.A. Grimm & Associates, Inc., hosted the seventh annual Innovators Showcase, an on-stage fireside chat where attendees get to know an innovator in the industry and discover insights from that individual’s experiences. This year’s subject, Andy Christensen, is founder and past president of Medical Modeling Inc.





 Considering that someone had to prove the technology out for
medical applications, Medical Modeling bought the S12 and set to work.
Considering that someone had to prove the technology out for
medical applications, Medical Modeling bought the S12 and set to work.

 Video
Video