The manufacturability inherent in 3D metal printing delivers even more.
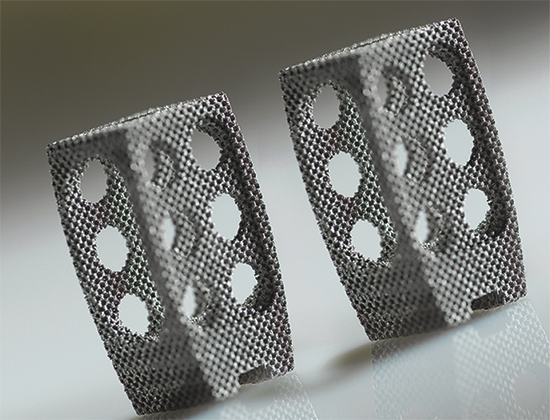
“The porous surfacing for osseointegration is a key factor because bone likes to adhere to something,” Van Deren says, noting that 3D printing enables production of porous structures, which is unique among metalforming and fabricating processes. “For example, in a hip implant, an asceptabular cup features an inverted bowl that is smooth on the inside and includes a porous coating on the concave side. The porous side locates toward the medial part of body, and the smooth inside part of the cup—post-process machined—articulates with the implanted femoral head. Spongy cancellous bone can grow into the porous crevices of the 3D-printed implant instead of just gliding over the smooth surface of a conventionally produced polished-metal implant. It gains purchase into the skeletal system of the patient at the correct site.”
 |
| Strong yet delicate weight-bearing joints, ankles are ideal candidates for 3D-printed metal implants. A host of designs currently are on the market. Photo courtesy of Concept Laser. |
The ability for existing bone to grow into porous surfaces of 3D-printed implants has been credited with cutting hip-implant recovery time from months to weeks. Spinal implants with the same porous surfaces that enable tissue to rapidly adhere and gain stability of the implant in the spinal column are another big driver for metal AM. The same is true with dental screws, using porous screw heads that allow for tissue adhesion.
And, beyond implants, 3D metal printing is used to produce instrument prototypes and power tools as well as structural-mobility products such as carts, tables, chairs and beds. Patient-specific reference models are another growing application for metal (and nonmetal) 3D printing.
“These can be printed based on radiographic imaging, and are used for presurgical planning and/or patient communication efforts,” says Van Deren. “It’s helpful in that a doctor has a tangible model in hand to discuss preoperative procedures with the patient, and also helpful when making decisions such as where to locate the point of entry or how much section of bone to remove.
|
Print-On-Demand for Patients on the Table?
“Many surgeons want print-on-demand for operating-table situations,” says medical-AM expert Shannon Van Deren, president of Layered Manufacturing and Consulting, Canton, MI.
But build challenges as documented in the accompanying feature article make that scenario unlikely anytime soon. In addition, designing a patient-specific item in short order poses a host of difficulties.
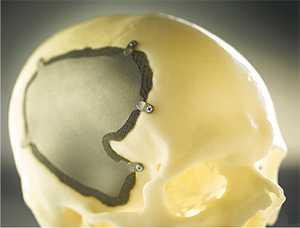 |
| This patient-specific cranial implant, designed and built for a recovering individual, may one day be printed on-demand for trauma victims. Photo courtesy of Concept Laser. |
“Right now we don’t have a way to convert radiographic images (CT scans or MRIs) to part files on the fly,” she says. “CT and MRI images are composed of many slices. First you need an engineer well-trained in converting such images to an STL file, for example. That takes special skill and unique software. In addition, clean-up is required. Scans contain floating artifacts and someone must determine if those might be bones or tissue, for example, and then all of the scans must be corrected and stacked in consecutive order to yield the patient’s anatomy in the STL file. This process, at best, may take a couple of days. From that point the STL file has to be prepped for build. Improper support on the build table or warping due to thermal conductivity will render an implant useless to the surgeon. We just are not at the point yet to accomplish this.
“But certainly there is demand for it,” Van Deren continues. “Every surgeon has mentioned this and AM technology providers are thinking about it. It’s quite a triumph that already we can convert CTs and MRIs into STL, as laborious as that process can be, and I expect that we will see more growth of capability. Maybe in five or 10 years this will be a reality for immediate, onsite applications.”
The technology investment alone required for print-on-demand is significant.
“Finding a skilled engineer to convert images to files can be tricky for a hospital,” explains Van Deren. “And, hospital officials will say that they want to bring a 3D printer inhouse, and see it as a $100,000 investment. But there are the engineers to pay for, and the software, and a machine operator and the material. And oh yeah, there’s all of the finishing equipment and related personnel.”
|
“Another example: Looking at an organ to see where an abnormality may be compromising function, and determining what to stay away from and what to remove during a procedure,” she continues. “Pre-operative models can create more positive outcomes for patients and reduce the chances of infection because surgery can be precisely planned beforehand. Also, the use of models for presurgical planning has gained traction as a tool to save time on the operating table, which significantly cuts costs for patients and hospitals.”
Porosity is the ‘Secret Sauce’
Getting back to implants, from one manufacturer to the next, porosity designs differ.
“Each manufacturer designs its own porosity recipe,” Van Deren says. “They’ll choose the pore shape and the strut size of that porosity, and choose the density, which refers to how close together the pores and struts reside. These designs are the result of clinical research that determines the rate of bone ingrowth as well as the body reception of bone ingrowth into their particular porosity recipe. Most companies will protect that information, as the porosity is their secret sauce. Titanium is just that until you’ve done something unique to it, and AM is the unique process that yields porosity.”
Overall designs differ as well from supplier to supplier. Spinal and dental implants are prime examples, with differing screws and geometric designs making no two look exactly alike. Again, research determines these designs.
Just as medical-device designs evolve based on research, the same is true for AM materials.
“Material suppliers have responded to the uptick in interest among AM suppliers,” says Van Deren.
“We see more metal alloys coming online for medical applications,” she adds. “Titanium and cobalt-chrome are widely used in the implant sector, for instance, and new formulations and new powder sizes (that help determine density and surface finish) are coming online.”
Standards and Regulations Galore
Of course, in a life-and-death industry, medical-AM manufacturers and their suppliers must adhere to a variety of stringent regulations, meet demanding standards and undergo rigorous certification processes.
The U.S. Food & Drug Administration (FDA) resides at the top of the watchdog list, and is heavily involved in the scrutiny of each new design and the process by which it is manufactured.
“More than 85 FDA-approved medical devices have been identified, which include implants, instrumentation and other patient-specific products, and that number is growing as more medical manufacturers adopt AM and more AM companies enter the field,” Van Deren says. “The FDA obviously has great intentions to protect us and allow us to enjoy the efficacy of medical devices, and it has seen that manufacturing is changing through the use of AM.
“The agency is accustomed to seeing submissions for 510(k) clearance (also known as Premarket Notification or PMN, which requires device manufacturers to notify the FDA of their intent to market a medical device or re-introduce a redesigned device at least 90 days in advance) for devices produced via conventional machining or injection molding,” she continues. “Now it is seeing submissions featuring AM and has developed a team to further study 3D printing. The FDA is working with equipment suppliers to understand how AM machines are validated at build, and then working with product manufacturers on validating their processes. The same is true for materials.”
FDA Offers Guidance
In 2016, the FDA published a guidance document addressing AM, Technical Considerations for Additive Manufactured Devices. In it, the FDA recognizes the value of AM, along with the concerns it brings.
“For medical devices, AM has the advantage of facilitating the creation of anatomically matched devices and surgical instrumentation by using a patient’s own medical imaging,” the document reads. “Another advantage is the ease in fabricating complex geometric structures, allowing the creation of engineered porous structures, tortuous internal channels and internal support structures that would not be easily possible using traditional (non-additive) manufacturing approaches. However, the unique aspects of the AM process, such as the layer-wise fabrication process, and the relative lack of medical-device history of devices manufactured using AM techniques, pose challenges in determining optimal characterization and assessment methods for the final finished device, as well as optimal process validation and acceptance methods for these devices.”
About Layered Manufacturing & Consulting Shannon Van Deren always had a love for the medical field. She’s parlayed that, following a career working for service bureaus, into her own business, Layered Manufacturing & Consulting, Canton, MI. As president, VanDeren consults businesses looking to enter or expand in the AM field. “Layered Manufacturing was born in an effort to be agnostic but well-informed,” she says. “We offer information, investigation, full adaptation and awareness of AM without an agenda to persuade a customer toward a single technology, material or service provider. We try to understand what they need and figure what’s needed to best yield their end product or needed technology.”
The company provides advice on designing for AM, and if a client needs parts on-demand, Van Deren has developed strategic partnerships and project-management services. On the medical side, Layered Manufacturing & Consulting counsels hospital systems on AM capabilities and technology.
www.layeredmanufacturingandconsulting.com |
FDA’s draft guidance, says Van Deren, “was an opportunity to show us in the field that FDA is taking us very seriously and is making progress in studying the technology and the outcomes, and what is necessary to have consistency in the quality of products entering the marketplace. As work proceeds, expect FDA directives within the next few years covering medical-device production via AM.”
Even though the FDA is in investigative mode, it is accepting submissions and offering approvals and denials on new products as well as redesigns of products currently on the market.
“I believe that the FDA is being responsible,” says Van Deren. “We all wish that things would move more quickly with regard to directives and the approval process, but the FDA is doing its due diligence and being attentive to the cause even in its learning phase.”
Validate, Validate, Validate
For those looking to navigate the FDA-approval process, Van Deren offers salient advice.
“Approval involves the material and process involved, the process technology, and the process flow,” she says, “and an applicant also must show clinical-trial efforts. There are so many checkmarks to meet along the way just to be ready for submission to the FDA. And, not only meeting FDA requirements, which qualifies end products, but those in medical manufacturing must comply with ISO 13485 medical certification. In short, manufacturers must validate the manufacturing floor and the process, the equipment, process flow, quality, machine maintenance, and post-processing of parts.”
Material usage across machines also earns close scrutiny from the FDA.
“The FDA appreciates a dedicated machine for, say, titanium,” Van Deren offers. “Machine suppliers may have to build and validate their equipment only for titanium. And, when a product design changes, validation restarts. Sure it’s a pain, but since we all may end up on a table getting the implants, we should appreciate the scrutiny.”
Certification and validation requirements can overlap for aerospace and medical manufacturing, Van Deren notes.
“If a manufacturer has AS 9100 certification in an aerospace facility and wants to prep for ISO 13485, a gap analysis likely will reveal that the gap is not so great,” she says. “Aerospace manufacturers already use titanium and cobalt-chrome, so they are familiar with and may have dedicated machines for these materials, which eases the transition. On the other hand, a service bureau building all types of parts that has not attained AS 9100 will have a lot to do, and will have to invest time and money.”
Given the research and clinical trials required as well as the regulatory steps, design of any medical device, especially implants, can take a couple of years. The manufacturing process itself is demanding, requiring post-processing that includes heattreating to prevent deformation, wire-EDM work to remove supports and then finish machining, which may entail CNC machining to capture tight hole tolerances. ASTM standards address passivation for medical devices, representing another finishing step. After all of that, sterile packaging is likely required.
Of course, everything is tracked in order to follow every step and every hand a device touches on the way to a patient. Lot and batch numbers can track material use as well as date and time of manufacture. Even the location on a build plate may be have to be tracked.
Bottom line, says Van Deren: “Medical devices require full traceability, and it takes time to build up processes that capture all of the information while being efficient and responsible.” 3DMP
See also: Concept Laser Inc., Layered Manufacturing and Consulting
Technologies:
 Lou Kren
Lou Kren