Aluminum Ramping Up
High-strength aluminum presents some of the same ductility and springback problems as HSS, along with higher costs, McClure says. These factors had been holding back the use of aluminum, but now that manufacturers are moving toward higher-strength materials, there is less of a barrier to using aluminum.
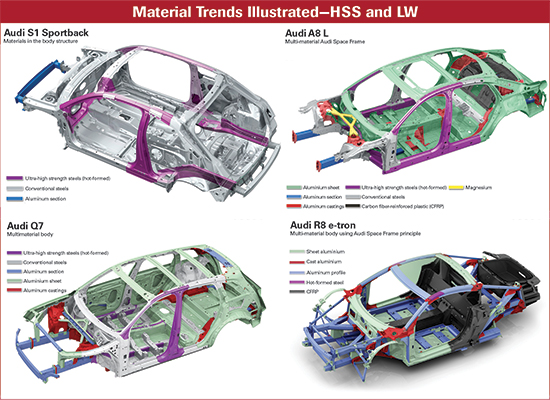 |
| Fig. 2—Many vehicle manufacturers are incorporating more HSS and aluminum into their vehicle frames. (Figure courtesy of Houghton International) |
“Aluminum is expanding into more applications,” at least for some vehicles, says Dianne Carmody, Americas marketing director, global product management adjacent businesses for Houghton International (Fig. 2).
Randy Sebastian, Houghton’s technical program manager, research and technology, concurs. He says that some semi-truck manufacturers have reduced the weight of their diesel engines by 50 percent by going from cast iron to compacted graphite iron for their engine blocks, but that this material is very difficult to machine. As a result, Sebastian says engine makers are moving toward aluminum for their gasoline engines. Among them--Mitsubishi and Cummins, who already have begun introducing aluminum diesel engines. Other aluminum engines are in the prototype stage.
Fluids Evolve
As materials and alloys change, the processes for working with them must change as well. Fluid formulators are looking for fluids with long service life to reduce downtime and increase productivity. Their customers are going toward faster speeds, using smaller sumps. Everyone wants low-foaming fluids that do not stain, emulsions that remain stable in hard water and additives that do not promote microbial growth (while still complying with regulations on allowable biocides). Not only that, but manufacturers are looking for compatibility with everything the fluid comes into contact with. “You have to look far downstream,” McClure says.
“All of the products have a place in the industry,” says Zhao. “North American and Asian companies use more water-based fluids, but European companies also are starting to move away from straight oils and toward water-based fluids.
|
Adhesives HSS and aluminum parts are more difficult to weld than mild steel. Epoxy structural adhesives are sometimes used in addition to or instead of welding in situations where welding is difficult. Epoxies are good sound deadeners and sealers, and they increase structural rigidity. 2015 automobiles typically had about 55 linear feet of structural adhesives. 2017 models have about 68 linear feet, and this is predicted to increase to 87 linear feet by 2020. Some high-end, aluminum-intensive vehicles contain as much as 600 linear feet of structural adhesives. Source: Ted McClure, Sea-Land Chemical Co. |
“Water-based fluids are complex packages, though, and require users to balance all the components,” Zhao continues. “Operations using high-strength materials generate more heat, and water-based fluids are better coolants than straight oils. Newer fluid technologies build in better detergency (prevention of particle agglomeration and coating formation on surfaces), dispersion and wetting, all needed for the lighter metals.”
Some operations still require straight oil metalworking fluids (MWFs), says Mark Soder, Houghton’s director of technical service, research and technology. Smaller parts and those requiring tight tolerances or a smooth surface finish are examples of this. Magnesium work often uses neat oil because it requires a lubricant that will not stain or release hydrogen gas. However, even here the trend is toward water-soluble fluids, for insurance reasons. Emulsion-based fluids are less flammable than neat oils and you do not have to evacuate the mist like you do with oils, he says.
High Temperatures, High Pressures
High-speed machining fluids for working with softer metals must have good cooling and lubricating properties to prevent excessive heat formation due to friction. Softer metals can expand and lose strength if the temperature rises too high. However, many of the new high-strength alloys must be heated to make them ductile enough to work with.
Aluminum alloys that were previously only used in the aerospace industry are beginning to be used in the automotive industry, McClure says (Fig. 3). The 6000- and 7000-series alloys, formed at elevated temperatures—500 to 750 F)—don’t always require a lubricant. When they do, the lubricant may include more solids, inorganics and phosphates. (More on no-lubricant scenarios later.)
Lubricants for the automotive industry have to perform at higher temperatures and pressures, McClure says, while maintaining compatibility with adhesives, cleaners, primers and welding. Straight oils don’t encounter the biological or hard-water problems that water-based fluids do, he adds, but they often become volatile at higher temperatures, producing fumes and smoke, and they may be prone to oxidation.
Oxidized lubricants are harder to clean off of parts, McClure adds. Lubricants and other fluids must not interfere with adhesives, including structural adhesives, which are seeing increasing use as a replacement for welding. This is an important research area, McClure says (see Adhesives).
Dave Slinkman, senior vice president, global research and technology for Houghton, notes that rolling HSS or aluminum sheet requires greater force, but at the same time body panels need a smoother surface finish than other parts might require. “We’ve had to make significant changes to the lubricity of our formulas to be able to work in those environments,” he says.
 |
| Fig. 3—Aluminum alloys that formerly were used only in aerospace applications are finding their way into automobile manufacturing. ©Can Stock Photo/Leaf |
Zhao says that working with lighter, stronger metals increases the need for boundary/EP lubrication additives. The hard surfaces of HSS, aluminum alloys and the titanium alloys used in the aerospace industry require a different lubrication mechanism. “We test additives by themselves and as parts of formulation packages for specific applications, and we use design of experiments to optimize our formulations,” he says.
Taming Rogue Ions
Hard-water cations (positively charged ions) can cause emulsions to separate, and they can leave mineral deposits on tools and workpieces. Calcium and magnesium are the usual suspects, but aluminum ions can cause hard water as well. Cast aluminum alloys contain magnesium, notes McClure, and magnesium is particularly hard on water-soluble cutting fluids. Formulations must contain additives that can stabilize emulsions in this environment.
Lightweight metals such as aluminum and magnesium corrode more easily than steel, and their protective oxide coatings are stable over a much narrower pH range than iron oxides. Semi-stable emulsions that deposit an oil coating on the workpiece are one option for preventing corrosion, though cleaning off the oil film is necessary before applying coatings or paints.
Even a small amount of corrosion can present a problem in the form of staining. Aluminum, magnesium and their alloys are prone to staining. Aluminum is a more reactive metal than steel, so processing fluids must contain corrosion inhibitors to prevent staining, says Hoon Kim, senior principal R&D scientist with Chemetall, Jackson, MI (see Corrosion Inhibitors: A Primer).
|
Magnesium 101
–Third most commonly used structural metal after steel and aluminum – Mainly used for making aluminum alloys – Lightest metal used in the production of structural components – Extremely high strength-to-weight ratio – Used for making wheels, radiator supports and engine blocks – Alloys among the easiest metals to machine – High thermal conductivity and dissipates heat rapidly – Sometimes machined dry without using any sort of metalworking fluid • Water-based fluids can stain magnesium more easily than they can aluminum or zinc.
|
Ferrous alloys corrode in neutral to acidic environments, but not in highly alkaline environments, because their surface oxide layers are stable at high pH. Aluminum readily forms a protective oxide layer, but the oxides are only stable in a fairly narrow pH region on either side of neutral. This can present a problem when MWFs are kept at a pH above 9 to protect expensive steel tools, Kim says. At this high pH, aluminum workpieces stain, and high-alkaline fluids can dissolve the protective aluminum-oxide layer as fast as it can form, so MWFs require corrosion inhibitors.
Processes that generate fresh metal surfaces by machining or grinding away the oxide layer require the use of fluids that protect these surfaces from direct contact with tool surfaces or chips to prevent welding or adhesion, often referred to as “sticking.” Because stainless steels, aluminum and titanium form a pure oxide skin, the sticking effect is more pronounced for these metals than for steel or copper.
Mild corrosion leaves a yellow or gold stain on aluminum. This can occur when using the right type of MWF but for too long, resulting in depletion of the corrosion inhibitor. Using MWFs meant for ferrous metals can cause more severe corrosion that leaves a gray or black stain. Even the right MWF can stain an aluminum workpiece if certain additives (triazine biocides, for example) raise the pH of the fluid too high.
Semi-Solids? Solids? No Lubricant?
Not every metalforming operation uses a lubricant, McClure says. HSS containing boron (in the gigapascal-tensile-strength range), formed at 1202-1562 F, are quenched in the die to form very strong parts. Conventional lubricants cannot withstand these temperatures.
Magnesium often is machined dry, without a cutting fluid, because it does not need the cooling or the lubricating effects. MWFs are sometimes used in the more difficult machining operations (e.g., deep-hole drilling) or work at high spindle speeds, but here the MWF is mainly a coolant, especially to keep the chips from igniting (see Magnesium 101).
Some aluminum-forming operations use semi-solid, half-hard lubricants, McClure says. Semi-solid or solid lubricants overcome some problems because they stay put rather than migrate within a coil, preserving an even coating, and they can provide excellent lubricity. However, these coatings can be costlier to apply and, like traditional fluids, must be compatible with assembly, cleaning and painting.
Adaptability Issues
Some fluid formulations advertised as multipurpose and suitable for a wide range of metals and applications may not live up to the one-size-fits-all claim. “It’s always a balancing act,” McClure says. You can optimize your formulation for one type of metal or one operation, but “users are reluctant to inventory too many different fluids in the plant.” Thus, it’s a trade-off between simplifying inventory and optimizing performance. “If it’s a critical operation,” he says, “you might use a fluid that’s specific to one type of material in a particular type of operation.”
A new lubricant must be approved for use before an OEM introduces it onto the factory floor, where making changes involves a long process, McClure says. Changes in the factory tend to be incremental to prevent issues in the manufacturing process. For example, fluids must be compatible not only with workpieces (which can have various surface treatments, including the galvanized coatings on HSS), but also perform well with the various tool materials and die coatings with which they come into contact (Fig. 4).
 |
| Fig. 4—Houghton chemist Scott Lay inspects an AHSS cup made with the state-of-the-art Valley Forge Labs 40-ton stamping press that the company uses to evaluate new technologies. (Figure courtesy of Houghton International) |
Environmental Considerations
Environmental considerations affect fluid formulations as well. Carmody sees a trend toward “minimum quantity lubrication” and away from “flood lubrication.” This approach typically requires a change in manufacturing equipment as well as different fluid formulations, she says, so operations generally do not adopt this approach until they are ready to replace their equipment.
Dry machining has proven successful in numerous operations, but MWFs still are required for applications that require cooling and lubrication, including titanium milling for aerospace applications or working with compacted graphite iron for engine parts. Using an MWF reduces tool wear (thus reducing tool replacement and disposal costs), and produces better parts by reducing residual stresses and dimensional errors, and improving surface finish. Fluids also allow processes to run at faster speeds without building up excessive heat. These factors can more than balance out the environmental impact of using fluids in the process.
Environmental considerations also affect what additives are used. For example, McClure says, conventional EP additives, including sulfurized, chlorinated and phosphorus-bearing additives, can react with steel but not necessarily with nonferrous metals, zinc-coated steels and tool coatings. OEM plants have gotten away from chlorine, he adds, but it is difficult to replace chlorinated fluids in some severe operations, including fineblanking. Also difficult, he says, is replacing chlorine for severe operations on some stainless steels as well.
Regulatory requirements differ from one region to another, says Slinkman. Older fluids, including those that contain chlorinated paraffin, may be acceptable in one region, but fluid suppliers in other regions may have to make significant changes to their products. Products for sale in Europe must comply with the Registration, Evaluation, Authorisation and Restriction of Chemicals (REACH) regulations.
“We have to conduct a complete review of our product lines to make sure that we are compliant,” Slinkman says. “We expect this to have a huge impact on the industry in terms of what products are available for these new uses.”
Often an old fluid that works well in one region of the world is heavily reformulated for sale in another region, Slinkman continues. “That’s a big change from what we’ve seen.” To keep up with the regulatory changes while reducing the amount of reformulation work, fluid development begins by finding “a basket of raw materials that we can use in five or six different regions,” he says.
|
Corrosion Inhibitors: A Primer Various classes of chemical compounds can prevent corrosion and staining on aluminum metal and alloys. Each has advantages and disadvantages. Inorganic compounds
Heterocyclic organic compounds
Sulfonates and phosphates
Amine carboxylates
Complex esters
Silicates
Silanes
Compounds with branched organic substituents
Amine-functionalized organosilicates
|
Environmental Considerations
Carmody notes that many of Houghton’s customers want products that they can use in most or all of their locations around the world. Sometimes, however, specialized regional formulations are required to accommodate differences in things such as water quality, she adds.
MWF Market
Competition and regulation come together to drive changes in MWFs, McClure says. Changes in fluid formulations, driven not only by new demands from parts manufacturers (who, in turn, are driven by marketplace and regulatory demands), also are pushed by new product offerings from the chemical companies’ R&D labs. “I hear from both sides,” says McClure.
It’s not enough just to watch overall industry trends, says Chuck Faulkner, Houghton’s product marketing manager for metalforming, forging and heattreatment. We watch it all,” he says. “Individual OEMs do things differently. We work with colleagues all around the world, and they all work with different materials and processes.”
In a 2016 press release, Gaia Franzolin, global marketing manager for Swedish specialty-oil manufacturer Nynas, cited a prediction for a 5.5-percent global increase in demand for private cars and light commercial vehicles over the next 5 years. Franzolin predicted a concurrent growth in demand for MWFs, driven in part by new materials such as the lightweight aluminum and titanium alloys for weight reduction and fuel efficiency. “Ultimately, it’s important to remember that technological advancements also bring the need for improved MWF performance (longevity and stability). And, their lower consumption will be compensated by higher costs and improved tool life,” she says.
Lubrication: Looking Ahead
Budai notes that the trend to reduce weight in automotive powertrains continues, with the downsizing of engines from V8 engines to V6 and I4 engines and the addition of turbocharging. The smaller turbocharged gasoline engines generate more heat, which raises the under-hood temperature. A few years ago, he says, OEM manufacturers were replacing steel engine parts with thermoplastics, but now some of the commonly used thermoplastics can’t always deal with the higher heat. So, aluminum and magnesium are, in some cases, seen as more cost-efficient alternatives for replacing the higher-priced, high-performance thermoplastics.
Kim sees a role for polymer composites; he notes that the aerospace industry uses carbon-fiber reinforced plastic (CFRP) composites, in addition to aluminum, titanium and HSS. Automaker BMW uses some CFRP composites in its passenger compartments but not for structural components, Kim says. Because CFRPs are so easy to form, the aerospace industry has moved from steel to aluminum and then toward CFRP. It is possible that the automotive industry could follow this trajectory as well.
As challenging as today’s high-speed, high-temperature manufacturing processes are, even bigger changes are on the horizon. Carmody notes that the aerospace industry is beginning to use 3D printing for prototyping and part production, and the medical industry already uses 3D printing with metals and composites to make joint replacements. Printed automotive components would require no processing fluids, and complex parts could be formed as one solid piece, with little to no need for machining, forming, stamping or other processes that generate heat and waste material.
The challenges involved in integrating these new materials and processes into efficient, reliable vehicles are becoming increasingly complex. Fluid formulators who work with the automotive industry clearly have their work cut out for them. MF
View Glossary of Metalforming Terms
Technologies: Lubrication, Materials






 OEMs are looking for strong materials with the desired degree of ductility and wear resistance. “We’re seeing more aluminum alloys containing titanium in specific engine components—power train valves, camshafts, pins, crankshafts, and parts such as exhaust and intake valves,” Budai says. His colleague, Yixing (Philip) Zhao, senior research scientist and innovation team leader, research and technology, at Houghton, adds that newer aluminum alloys have higher strength, different compositions and harder surfaces. The aerospace industry is the biggest user of titanium right now, Zhao says, but the knowledge that fluid developers gain in developing lubricants for this market eventually transfers to other industries.
OEMs are looking for strong materials with the desired degree of ductility and wear resistance. “We’re seeing more aluminum alloys containing titanium in specific engine components—power train valves, camshafts, pins, crankshafts, and parts such as exhaust and intake valves,” Budai says. His colleague, Yixing (Philip) Zhao, senior research scientist and innovation team leader, research and technology, at Houghton, adds that newer aluminum alloys have higher strength, different compositions and harder surfaces. The aerospace industry is the biggest user of titanium right now, Zhao says, but the knowledge that fluid developers gain in developing lubricants for this market eventually transfers to other industries.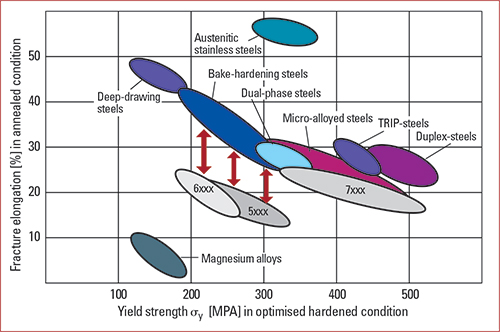


 White-paper
White-paper