Farsoon-Printed Spine-Cage Implants Earn Chinese Clearance
March 8, 2021Comments
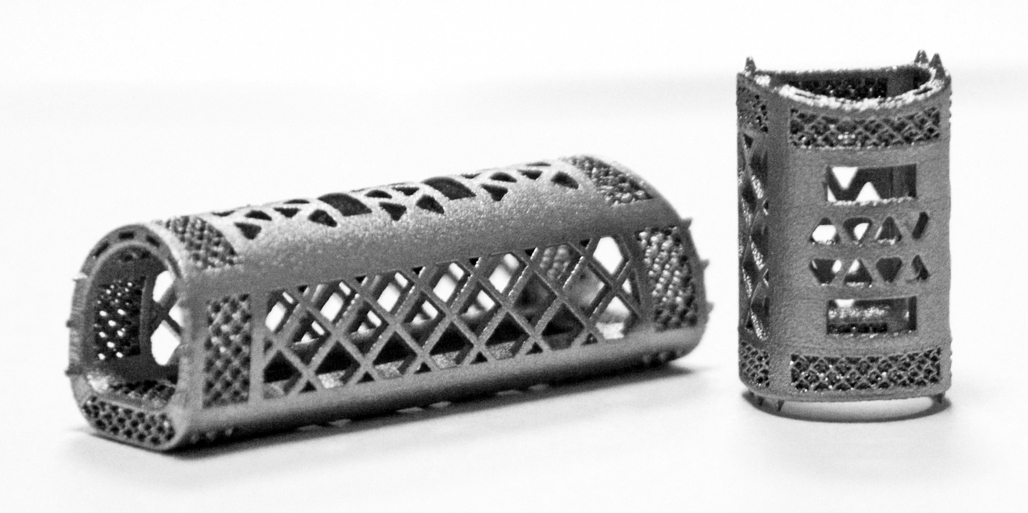 Huaxiang Group, a provider of 3D-printed medical components, and Dr. Wang Wenjun’s Spinal Surgery team from First Affiliated Hospital of University of South China, recently received Category 3 medical-device clearance from China’s National Medical Products Administration (NMPA), for its porous spine-fusion cages, produced on metal-additive manufacturing (AM) systems from Farsoon Technologies, represented in North America by Farsoon Technologies Americas, Round Rock, TX. This marks the first approved orthopedic implants built via metal laser powder-bed fusion technology in China, according to Farsoon officials.
Huaxiang Group, a provider of 3D-printed medical components, and Dr. Wang Wenjun’s Spinal Surgery team from First Affiliated Hospital of University of South China, recently received Category 3 medical-device clearance from China’s National Medical Products Administration (NMPA), for its porous spine-fusion cages, produced on metal-additive manufacturing (AM) systems from Farsoon Technologies, represented in North America by Farsoon Technologies Americas, Round Rock, TX. This marks the first approved orthopedic implants built via metal laser powder-bed fusion technology in China, according to Farsoon officials.
This titanium cage represents an advanced solution over traditional trimmed cages and anatomical cages for treatment of spinal conditions such as degeneration, fractures, deformities and tuberculosis. It is identified as one of the key medical-implant products identified by the 3D Printing Technology Application of Personalized Implant Device project headed by China’s leading hospitals, research institutes, material manufacturers and 3D-printing specialists. NMPA approval recognizes project partners’ combined knowledge in medical-grade material, AM, design software, medical-device development and clinical application.
“This is a ground-breaking day for metal 3D printing in the Chinese medical market,” says Li Xinghua, Huaxiang Group’s head of marketing. “I’m grateful for joining a such strong team with Dr. Wang and Farsoon Technologies for this journey. We are very proud to contribute our effort to future patient-tailored spinal implant surgeries with the application of selective-laser-melting technology.”